
Register your product and get support at / 请登录以下网址并得到相应的帮助
www.philips.com/welcome


1
2
10
98
543
76
1

2 3 4 5
6
7 8 9
10 11 12 13
14 15 16 17
18 19 20 21

6
Introduction
This booklet contains important information on the PulseRelief device.
The complete user manual for this device is included in the app and can
also be downloaded from www.philips.com/support.
Read chapter ‘Contra-indications, reactions and side effects’ and chapter
‘Important safety information’ before you start using the device.
Intended use
The PulseRelief is intended to be used by adult consumers who
experience mild to moderate chronic pain or acute post-surgical pain.
The purpose of this device is to provide pain relief through
transcutaneous electrical nerve stimulation (TENS). You can also use it for
electrical muscle stimulation (EMS). The device is suitable for home use.
If you recently had surgery or are in the care of a doctor, do not use this
device without consulting your doctor rst.
Do not place the electrodes over open wounds or rashes.
Important safety information
Contra-indications
Do not use the device if:
- you have a cardiac pacemaker, implanted
debrillator or other implanted metallic or
electronic device (e.g. a drug delivery system),
as use in these cases could cause electric shock,
burns, electrical interference, or even death.
- you have a heart disease.
- you have epilepsy.
- you are in the rst trimester of your pregnancy, as
the effects of TENS on the development of the
foetus are as yet unknown. Avoid use over the uterus
during pregnancy to reduce the risk of inducing
ENGLISH

labour. If you are pregnant, always consult your doctor
or midwife before use.
- you have a cognitive impairment.
- you have venous or arterial thrombosis or
thrombophlebitis. If you use the device for EMS,
there is a risk of mobilising the blood clot.
- you have undiagnosed pain, unless your doctor
recommends use of this device.
Reactions
Stop using the device and consult your doctor if the
pain worsens or if you experience adverse reactions
from the device.
Possible adverse reactions may include the following:
- skin irritation beneath the electrodes, although the
gel used on the electrodes is not known to cause
allergic reactions
- burns beneath the electrodes
- headaches or other painful sensations
- nausea or feeling faint
Side effects
- After EMS, some muscle soreness may occur some
time after the treatment.
- Some reddening of the skin under or around the
electrodes may occur during and shortly after EMS
treatment. Skin reddening usually disappears within
two hours after treatment. If the skin continues to be
red for more than a day, please consult your doctor.
ENGLISH 7

Warnings
General warnings
- Read this user manual carefully and always adhere
to the treatment instructions.
- Stop using the device and consult a doctor if the
pain worsens.
- This is a medical device. Keep the device out of the
reach of children. Children may not be able to use it
according the instructions in this user manual. This
device should not be used on or by children.
- This appliance is not intended for use by adults with
reduced sensory or mental capabilities. They may
not be able to use it according the instructions in
this manual and they may become agitated by the
treatment.
- Do not use the device on children, as the device is
not designed to use on children.
- Do not modify the device or the electrodes. This
could cause improper functioning.
- Only use the adapter supplied to charge the device.
Use of another adapter may cause a hazardous situation.
- This device is designed for use by and on a single
adult person.
Do not apply to or treat the following
parts or areas
- Stimulation on the sides of the neck over the
carotid sinus may have severe adverse effects on
your heart rhythm or blood pressure.
ENGLISH8

- Stimulation on the front of the neck may cause
severe muscle spasms that could close your airway
and result in breathing difculties.
- Do not apply stimulation across your chest because
the introduction of electrical current into the chest
may cause rhythm disturbances to your heart, which
could be lethal.
- Stimulation across the head or on opposite sides of
the head must be avoided, as the effects on the
brain are unknown.
- Do not place the electrodes on skin that is not
intact, clean or healthy. Open, irritated or otherwise
damaged skin may lead to the delivery of too much
current to the area, which may potentially cause burns.
- If you recently had surgery, do not place one or both
electrodes on the cut. Place the electrodes at a distance
of 3 to 5 cm from the cut or along the proximal
nerves or near the spine where the skin is intact.
- Do not place the electrodes near cancerous lesions,
as this may have a negative impact on the cancerous
lesion.
- Do not place the electrodes on areas of skin that
lack normal sensation. This may cause you to be
unaware of the high intensity current administered,
which may result in electrical skin burns.
- Do not place the electrodes over open wounds or
rashes. Open wounds may lead to the delivery of
too much current to the area, potentially leading to
ENGLISH 9

burns. It might also cause substances in the
electrode to enter the skin.
- Do not place the electrodes over swollen, red,
infected or inamed areas or skin eruptions
(e.g. phlebitis, thrombophlebitis and varicose veins).
Stimulation must not be applied to areas with
known thrombosis or thrombophlebitis, as
stimulation may increase circulation, resulting in
a greater risk of emboli.
- Do not place the electrodes inside body cavities,
e.g. the mouth. This device is not designed for
internal application.
- For EMS, only place the electrodes on healthy,
uninjured muscles.
Do not use the device in the following
conditions
- Do not use the device if you have recently had
surgery or if you are in the care of a doctor and
have not consulted your doctor rst.
- Do not use the device if you have a tendency to
bleed internally due to any impact or injury.
- Do not use EMS stimulation to contract a muscle
if contraction of the muscle may disrupt healing.
For instance, if the muscle or tendon is torn,
muscle contraction may exacerbate the tear, just like
a voluntary contraction. This may also be the case
after a recent surgical procedure or following acute
trauma or fracture. Muscle contraction in case of
tendinitis may also worsen symptoms.
ENGLISH10

- Do not use the device while driving, operating
machines or performing any other activity in which
electrical stimulation can put you at risk of injury.
- Do not use if you are likely to fall asleep during the
treatment, as this may cause you to sense injuries
too late. If you use the device at bedtime, set the
timer to make it switch off automatically
- Do not use while in the bath, shower or swimming pool.
This increases the risk of electric shocks and burns to
the skin.
- Do not apply stimulation in the presence of electronic
monitoring equipment (e.g., cardiac monitors,
ECG alarms), which may not operate properly when
the electrical stimulation device is in use.
- Do not use the device when you are connected to
high-frequency surgical equipment. This may result in
burns on the skin under the electrodes and may
damage the device.
- Do not use the device within less than 1 metre
from shortwave or microwave medical equipment.
Close proximity to this equipment may cause
unstable device output.
- Portable RF communications equipment (including
peripherals such as antenna cables and external
antennas) should be used no closer than 30 cm
(12 inches) to any part of the device including
cables specied by the manufacturer. Otherwise,
degradation of the performance of this equipment
could result.
ENGLISH 11

- Use of accessories, transducers and cables other
than those specied or provided by the
manufacturer of this equipment could result in
increased electromagnetic emissions or decreased
electromagnetic immunity of this equipment and
result in improper operation.
Precautions
- If you had surgery recently, consult your doctor
before you use the device. Using the device may
disrupt the healing process.
- Use Burst TENS programs while resting, as these
programs can result in muscle contraction in the
treatment area.
- Make sure that you end the treatment in the Philips
Treatment App or press the on/off button on the
device before you remove the device or the
electrodes. If you do not end the treatment, you
may experience an unpleasant sensation in your
ngers when you touch the magnets. This sensation
is not harmful, but can be unpleasant.
- Only use this device with the adapter, cord and
accessories recommended by the manufacturer.
- Always check the device and the electrodes for
damage before use. Do not use the device or an
electrode if it is damaged.
- The device is not waterproof. Do not use the
device in wet surroundings and prevent it from
getting wet.
ENGLISH12

- Although you can use the device indoors and
outdoors, it does not withstand all weather
conditions.
- The device does not withstand high and low
temperatures. Please check the operating conditions
in chapter ‘Specications’.
- The electrodes have a limited shelf life. Please check
the packaging for the use-by date prior to use.
Do not use electrodes whose use-by date has expired.
For ordering replacement electrodes, see chapter
‘Ordering accessories’.
- Do not use plaster or tape to attach the electrodes
to the skin.
- Always use and store the electrodes according to
the instructions in chapters ‘Using the device’ and
‘After use’.
- If the device does not function as described here,
stop using it and contact the Consumer Care Centre.
For contact details, see chapter ‘Guarantee and
support’.
Compliance with standards
- The device meets the relevant standards for this
type of Class IIa electrical medical appliance and
appliances using electrical stimulation for home use.
- This Philips appliance complies with all applicable
standards and regulations regarding exposure to
electromagnetic elds.
ENGLISH 13

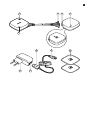
Product overview (Fig. 1)
1 On/off button
2 Status indicator
3 Detachable connector
4 Micro-USB socket of device
5 Battery indicator
6 Adapter
7 Socket for standard USB plug
8 Standard USB plug
9 Micro-USB plug
10 Self-adhesive hydrogel electrodes
Replacement
Electrodes
Replace the electrodes if:
- they are damaged or torn.
- they are past the use-by date indicated on the resealable bag.
- they have lost their adhesive power. Never use plaster or tape to
attach them to your skin.
- when stimulation is uncomfortable, i.e. when you experience an
unpleasant stinging or biting sensation.
Note: Always replace the electrodes with electrodes recommended for this
device by the manufacturer. You can order new electrodes at
www.philips.com.
Guarantee and support
Your device has been designed and developed with the greatest possible
care to guarantee an expected service life of 5 years.
If you need service or information or if you have a problem, please visit
our website at www.philips.com/support. You can also contact the
Philips Consumer Care Centre in your country. Its telephone number and
contact URL is:
- United Kingdom: 0844 338 04 89 (5p/min. from a BT landline,
other landline and mobile providers may charge more)
- Hong Kong: 852 2619 9663 (free of charge for landline calls).
ENGLISH14

Guarantee restrictions
The terms of the guarantee do not cover the electrodes, as the
electrodes are subject to wear and have to be replaced regularly.
Manufacturer’s legal address
Philips Consumer Lifestyle B.V.
Tussendiepen 4, 9206 AD Drachten, Netherlands
Recycling
- This symbol on a product means that the product is covered by
European Directive 2012/19/EU (Fig. 2).
- This symbol means that the product contains a built-in rechargeable
battery covered by European Directive 2006/66/EC which shall not be
disposed of with normal household waste. Follow the instructions in
section ‘Removing the rechargeable battery’ to remove the battery (Fig. 3).
- Follow your country’s rules for the separate collection of electrical and
electronic products and rechargeable batteries. Correct disposal helps
prevent negative consequences for the environment and human health.
- All plastic parts are marked with recycle symbols (Fig. 4).
Removing the rechargeable batteries
Note: Always remove the rechargeable batteries before you hand in the device
at an ofcial collection point. If you have trouble removing the batteries, you
can also take the device to a Philips service centre, which will remove the
battery pack for you and will dispose of it in an environmentally safe way.
1 Make sure the rechargeable batteries are empty. Switch on the
device as often as is necessary until you are no longer able to
switch it on because the batteries have run out completely.
2 Insertthetipofaat-headscrewdriverbetweenupperandlower
shellatthemicro-USBsocket.
Note: This action destroys the device and must only be executed if you intend
to discard the device.
3 Turn the screwdriver to separate the two shells.
Note: You may have to insert and turn the screwdriver a few times to break
the two shells loose from each other.
ENGLISH 15

4 When the shells have come apart, use the screwdriver to remove
the batteries. Dispose of the plastic parts and the batteries
separately according to local rules
Specications
Model PR3840
Rated voltage(V) - adapter 100-240 ~ 150mA
Rated frequency (Hz) - adapter 50-60
Rated output - adapter
5V , 500 mA
Class Medical device Class IIa
Ingress of water - device IP22
Battery type - device Li-Ion
Adapter The adapter is specied as part
of the medical system.
Pulse output parameters
Frequency range 1-100 Hz
Pulse duration 60-350 µs for programs 1 to 20
Maximum current output 60mA at 500-1000 Ohm
Maximum output voltage 60V
Current pulse shape
Biphase symmetrical (net current 0 DC)
Operating conditions
Temperature from +5°C to +40°C
Relative humidity from 15% to 93% (non-condensing)
Atmospheric pressure from 700hPa to 1060hPa
ENGLISH16

Storage and transport conditions
Temperature - electrodes from 0°C to +40°C
Temperature - device from -10°C to +50°C
Relative humidity less than 93% (non-condensing)
Atmospheric pressure from 700hPa to 1060hPa
Note: If you want to store the electrodes for more than a month,
keep them at temperatures between +5°C and +27°C.
Note: Do not store the electrodes in the freezer or refrigerator. Do not expose
them to high temperatures, nor immerse them in water, or leave them
outside of the plastic bag.
Explanation of symbols
Symbols on the adapter and the device
- This symbol indicates that the adapter is double insulated (Class II),
according to IEC 60601-1 (Fig. 5).
- This symbol means: Do not throw away with the normal household
waste. For further instructions, see chapter ‘Recycling’ (Fig. 2).
Symbols on the device
- This is the symbol for serial number. This symbol is followed by the
manufacturer’s serial number (Fig. 6).
- This is the symbol for reference number. This symbol is followed by
the manufacturer’s reference number, which is the type number of
the device (Fig. 7).
- This symbol on the device means: protected against access to
hazardous parts with a nger and against vertically falling water drops
when tilted up to 15 degrees (Fig. 8).
- This symbol means that the part of the device that comes into
physical contact with the user (also known as the applied part)
is of type BF (Body Floating) according to IEC 60601-1.
The applied parts are the electrodes (Fig. 9).
ENGLISH 17

- This symbol means that this device emits non-ionising radiation.
All devices and systems that include RF transmitters or that
intentionally apply RF electromagnetic energy must have a label with
this symbol (Fig. 10).
- This symbol means: caution TENS output. You nd this symbol near all
electrode connections (Fig. 11).
- This symbol means ‘Manufactured by’ and appears next to the
address of the legal manufacturer (Fig. 12).
- This symbol means: Conforms to EC Directives. CE stands for ‘Conformité
Européenne’. 0344 is the number of the notied body (Fig. 13).
- This symbol means: read the user manual before you start using
the device (Fig. 14).
- This symbol is the mark that identies the standby button (Fig. 15).
- This is the pulse symbol. It appears next to the status indicator (Fig. 16).
- This is the battery symbol. It appears next to the battery indicator (Fig. 17).
Symbols on the adapter
- This symbol means: alternating current (Fig. 18).
- This symbol means: direct current (Fig. 19).
- This symbol means: Conforms to EC Directives. CE stands for
‘Conformité Européenne’ (Fig. 20).
- This symbol means: may only be used indoors (Fig. 21).
- This symbol means: USB connector (Fig. 22).
- This symbol means: micro-USB connector (Fig. 23).
- This symbol is the certication logo that includes identication of the
accredited compliance test house. (Fig. 24)
- This symbol is the certication mark for technical equipment.
The GS mark is based on the German Equipment and Product
Safety Act (Fig. 25).
- This symbol shows that the no-load power CEC (California Energy
Commission) Efciency Level is V (ve) in order to meet EU requirements
(Fig. 26).
ENGLISH18

Symbols on electrode bag
- This symbol means: Do not use while driving, operating machines or
performing other activities that may present a risk of injury (Fig. 27).
- This symbol means: Do not use damaged or worn electrodes or
electrodes that have lost their adhesive power (Fig. 28).
- This symbol means: The electrodes should be used by one person
and not shared with others (Fig. 29).
- This symbol means: Do not place the electrodes on top of each
other or so close to each other that they touch each other (Fig. 30).
- This symbol means: Do not place electrodes on your head or on
your neck (Fig. 31).
- This symbol means: Do not place the electrodes on your chest (Fig. 32).
- This symbol means: Do not put the electrodes on open wounds,
cancerous lesions, rashes and infected or inamed skin. If you recently
had surgery, do not place electrodes on the cut (Fig. 33).
- This symbol indicates the minimum and maximum storage temperature
for electrodes if they are stored for more than a month (Fig. 34).
- This is the symbol for ‘use by’. It indicates after which date the
electrodes must not be used anymore (Fig. 35).
Symbols on the package
- This symbol means that the package and its contents can be stored at
temperatures between -10°C and +50°C (Fig. 36).
- This symbol means that the 2-year worldwide guarantee applies to
this device (Fig. 37).
- This symbol means that the device contains a Li-Ion battery (Fig. 38).
- This symbol refers to the Green Dot scheme, which is covered under
the European Packaging and Packaging Waste Directive (94/62/EC).
Under this scheme, industry has to pay for recycling the packaging
materials of consumer goods (Fig. 39)
Electromagnetic emissions and immunity
Electromagnetic Compatibility (EMC)
The PulseRelief device is approved according to EMC safety standard
EN 60601-1-2. It is designed to be used in typical domestic or clinical
environments.
ENGLISH 19

簡介
本手冊包含 PulseRelief 裝置的重要資訊。裝置的完整使用手冊隨
附於應用程式之內,也可以從 www.philips.com/support 下載。
適合用途
PulseRelief 專供有輕微到中度慢性疼痛問題,或是急性術後疼痛
問題的成人消費者使用。本裝置旨在透過經皮神經電刺激 (TENS)
緩解疼痛,您也可以用來進行肌肉電刺激 (EMS)。本產品適合在
家使用。
禁忌症狀、反應和副作用
禁忌症狀
如您有下列情況,請勿使用本裝置:
- 體內裝有心律調整器、植入心臟除顫器或其他金屬或電子裝置
(例如藥物釋放系統),在此情況下使用本裝置,恐會造成電擊、
灼傷、電氣干擾,甚至是死亡。
- 有心臟疾病。
- 患有癲癇。
- 妊娠第一期的孕婦。由於目前仍不確定 TENS 是否會對胎兒發
展有所影響,因此懷孕期間請避免用於子宮,以減少引發分娩
的風險。若您是孕婦,請務必於使用前諮詢您的醫師或助產士。
- 有認知障礙。
- 有靜脈或動脈血栓或血栓性靜脈炎。如果您使用本裝置進行
EMS (肌肉電刺激),可能會造成血塊移動。
- 若有未確診的疼痛,請勿使用,除非您的醫師建議使用本裝置。
反應
如果使用裝置時產生不良反應,請停止使用裝置並諮詢您的醫師。
可能發生的不良反應包括下列項目:
- 黏貼電極片部位出現皮膚過敏,雖然電極片使用的凝膠並無已
知會引起過敏反應的成份
- 黏貼電極片部位出現肌膚灼傷
- 頭痛或其他疼痛感
- 噁心或暈眩
繁體中文20
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
ページが読み込まれています...
-
 1
1
-
 2
2
-
 3
3
-
 4
4
-
 5
5
-
 6
6
-
 7
7
-
 8
8
-
 9
9
-
 10
10
-
 11
11
-
 12
12
-
 13
13
-
 14
14
-
 15
15
-
 16
16
-
 17
17
-
 18
18
-
 19
19
-
 20
20
-
 21
21
-
 22
22
-
 23
23
-
 24
24
-
 25
25
-
 26
26
-
 27
27
-
 28
28
-
 29
29
-
 30
30
-
 31
31
-
 32
32
-
 33
33
-
 34
34
-
 35
35
-
 36
36
-
 37
37
-
 38
38
-
 39
39
-
 40
40
-
 41
41
-
 42
42
-
 43
43
-
 44
44
他の言語で
関連論文
-
Philips 55PFL6300/T3 ユーザーマニュアル
-
Philips 43PUD6701/30 ユーザーマニュアル
-
Philips 50PUD6172/30 ユーザーマニュアル
-
Philips 43PFD5773/30 取扱説明書
-
Philips 32PHD5773/30 取扱説明書
-
Philips 55PUD6654/30 ユーザーマニュアル
-
Philips 58PFL8900/T3 ユーザーマニュアル
-
Philips 55PFL8300/T3 ユーザーマニュアル
-
Philips PR3840/00 ユーザーマニュアル
-
Philips PR3820/00 Product Datasheet














































